
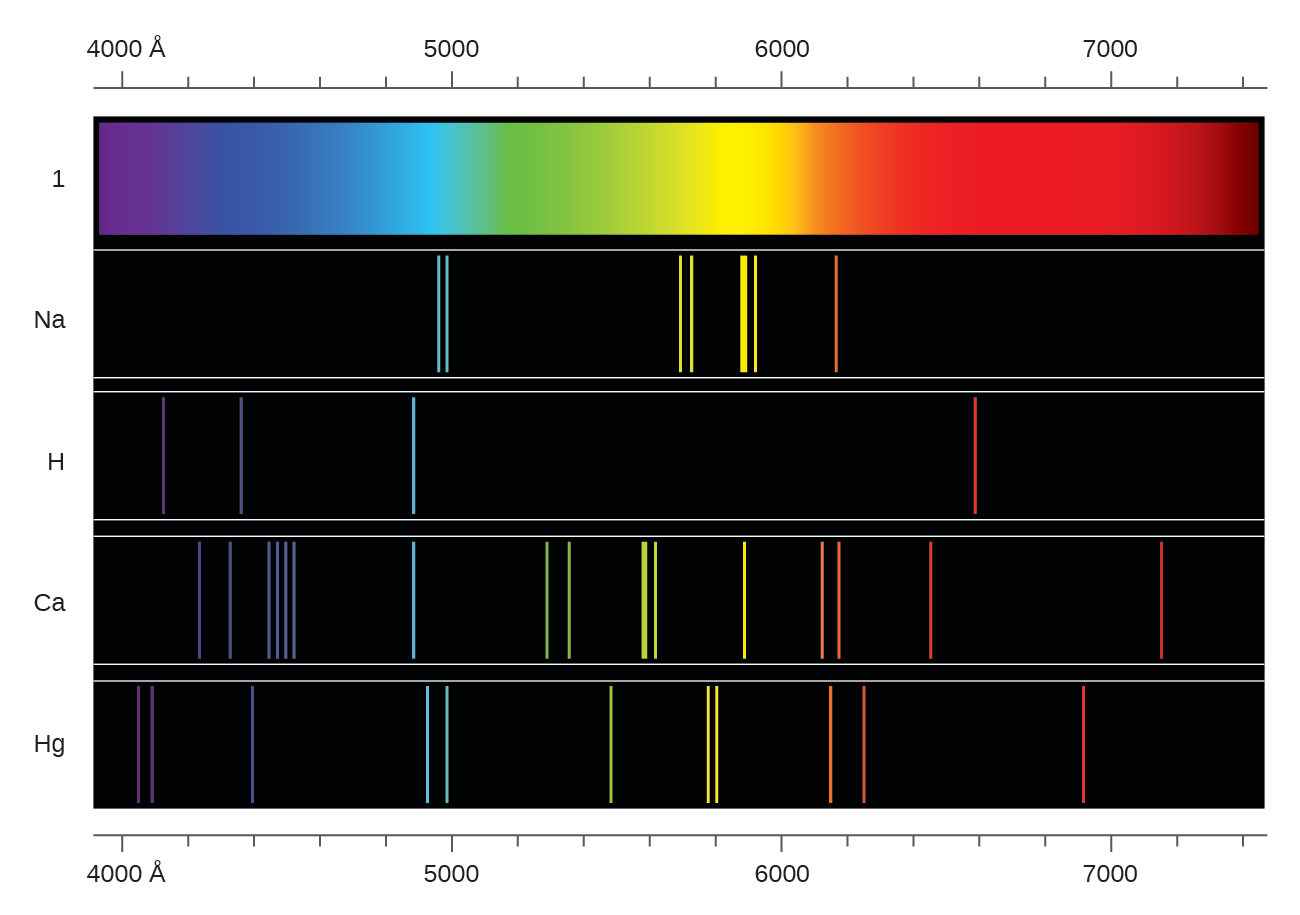
Thing with hydrogen, you don't see a continuous spectrum. So that's a continuous spectrum If you did this similar

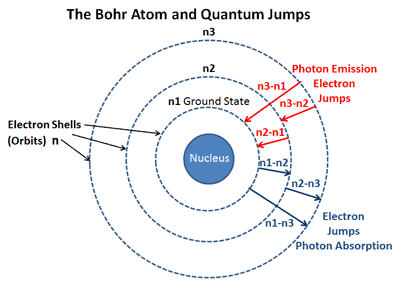
It's continuous because you see all these colors right next to each other. Like this rectangle up here so all of these differentĬolors of the rainbow and I'm gonna call thisĪ continuous spectrum. And so if you did this experiment, you might see something Of light through a prism and the prism separated the white light into all the differentĬolors of the rainbow. I'm sure that most of you know the famous story of Isaac Newton where he took a narrow beam of light and he put that narrow beam We identify the H in the star by seeing its characteristic absorption spectrum. The absorption spectrum is like the negative of the emission spectrum, it has dark lines where the emission spectrum has light. The absorption spectrum is what's left after the white light passes through the outer layers of the star, where the pressure is lower and the protons can join with the electrons to make complete H atoms. When astronomers study distant stars they actually look at ABSORPTION spectra rather than emission spectra. The sun radiates like a so-called "black body" with temperature around 5700K. The interactions between the particles create a different, much larger set of energy levels that permit a continuous rather than discrete spectrum. Also, you have enormous pressure, which forces all of the atoms to interact with one another in a way that they don't when they are in low-pressure gas form. In other words, you have a lot of H nuclei (ie protons) and a lot of electrons, and they aren't bound together to make a nice simple H atom. For one thing, the temperature is very high, so the atoms are ionized. The sun is not just a big ball of H atoms loosely floating around. To view the spectrum we need hydrogen in its gaseous form, so that the individual atoms are floating around, not interacting too much with one another. The discrete spectrum emitted by a H atom is a result of the energy levels within the atom, which arise from the way the electron interacts with the proton.
Infrared photons are invisible to the human eye, but can be felt as "heat rays" emitted from a hot solid surface like a cooling stove element (a red-hot stove or oven element gives off a small amount of visible light, red, but most of the energy emitted is in the infrared range). However, all solids and liquids at room temperature emit and absorb long-wavelength radiation in the infrared (IR) range of the electromagnetic spectrum, and the spectra are continuous, described accurately by the formula for the Planck black body spectrum. metals like tungsten, or oxides like cerium oxide in lantern mantles) include visible radiation.
Because solids and liquids have finite boiling points, the spectra of only a few (e.g. Because the electric force decreases as the square of the distance, it becomes weaker the farther apart the electric charged particles are, but there are many such particles, with the result that there are zillions of energy levels very close together, and transitions between all possible levels give rise to continuous spectra. The second case occurs in condensed states (solids and liquids), where the electrons are influenced by many, many electrons and nuclei in nearby atoms, and not just the closest ones. During these collisions, the electrons can gain or lose any amount of energy (within limits dictated by the temperature), so the spectrum is continuous (all frequencies or wavelengths of light are emitted or absorbed).
ATOMIC SPECTRA LINE SPECTRA FREE
The first occurs, for example, in plasmas like the Sun, where the temperatures are so high that the electrons are free to travel in straight lines until they encounter other electrons or positive ions. Continuous spectra (absorption or emission) are produced when (1) energy levels are not quantized, but continuous, or (2) when zillions of energy levels are so close they are essentially continuous. in outer space or in high-vacuum tubes) emit or absorb only certain frequencies of energy (photons). Line spectra are produced when isolated atoms (e.g.


 0 kommentar(er)
0 kommentar(er)
